Week 247
Protein Engineering, RNA 3D Structure Prediction, Blood Pressure Monitoring, AI Workload Impact
We are moving our newsletter to Substack for a better experience!
In Week #247 of the Doctor Penguin newsletter, the following papers caught our attention:
1. Protein Engineering. Understanding the relationship between a protein's amino acid sequence and its biological function is crucial for advancing biology and developing new therapeutics. While protein language models (PLMs) can design novel proteins, their zero-shot predictions often show limited success, with engineered proteins exhibiting lower or comparable activity relative to natural wild-type sequences. This limitation stems from PLMs' inability to generalize to new contexts. To overcome this challenge, recent studies have combined two approaches: directed evolution, which mimics natural selection by iteratively selecting beneficial mutations in the lab, and active learning, which uses algorithms to strategically select a small set of unlabeled data for annotation to improve predictions.
Jiang et al. developed EVOLVEpro, a few-shot active learning framework that combines PLMs with regression models to guide directed protein evolution. Through active learning, the system iteratively tests small sets of predicted mutations and uses the experimental data to refine subsequent predictions. Testing across six proteins relevant to medical applications, EVOLVEpro achieved up to 515-fold improvements in desired properties while minimizing wet lab experimentation compared to conventional methods. The system is able to select highly active single mutants out of more than 16,000 possible sequences and multi-mutants from more than 780 billion possible sequences. Moreover, EVOLVEpro prompting only uses protein sequences and does not require structural information, expert knowledge, or prior data. Structural analysis of successful mutations revealed multiple mechanisms of activity improvement. The results highlight a fundamental challenge in protein engineering: while PLMs trained on evolutionary sequence data can capture broad patterns in protein structure and function, they may not directly optimize for specific desired activities since natural evolution operates under different constraints than engineered protein optimization. EVOLVEpro addresses this limitation by combining PLMs with targeted experimental testing, offering an efficient pathway for engineering proteins with enhanced therapeutic and biotechnology applications.
Read paper | Science
2. RNA 3D Structure Prediction. There is a speed and accuracy trade-off in current RNA structure prediction methods. Traditional approaches fall into two categories: template-based methods like ModeRNA and RNAbuilder, which are constrained by limited template libraries, and de novo prediction methods like FARFAR2 and SimRNA, which achieve higher accuracy but require computationally intensive sampling. Recent deep learning approaches using multiple sequence alignments (MSAs) have improved prediction accuracy. However, these methods require extensive database searches to construct MSAs, making them time-consuming to use.
Shen et al. developed RhoFold+, a language model-based deep learning pipeline for predicting 3D structures of single-chain RNA from sequence data. The system combines representations from a language model pretrained on 23.7 million RNA sequences with a selective set of MSAs (limited to 256 sequences), processing both through transformer networks to predict RNA structures. This approach addresses two key challenges: the limited availability of structural data (RNA structures comprise less than 1% of the Protein Data Bank) and the speed-accuracy trade-off in RNA structure prediction. RhoFold+ generates predictions in approximately 0.14 seconds while maintaining competitive accuracy - achieving a mean RMSD of 4.02Å on RNA-Puzzles targets compared to 6.32Å for the next best method. Testing across different RNA families and types demonstrated RhoFold+'s ability to predict structures of previously unseen RNA molecules. Although designed primarily for 3D structure prediction, RhoFold+ can also accurately predict RNA secondary structures.
Read Paper | Nature Methods
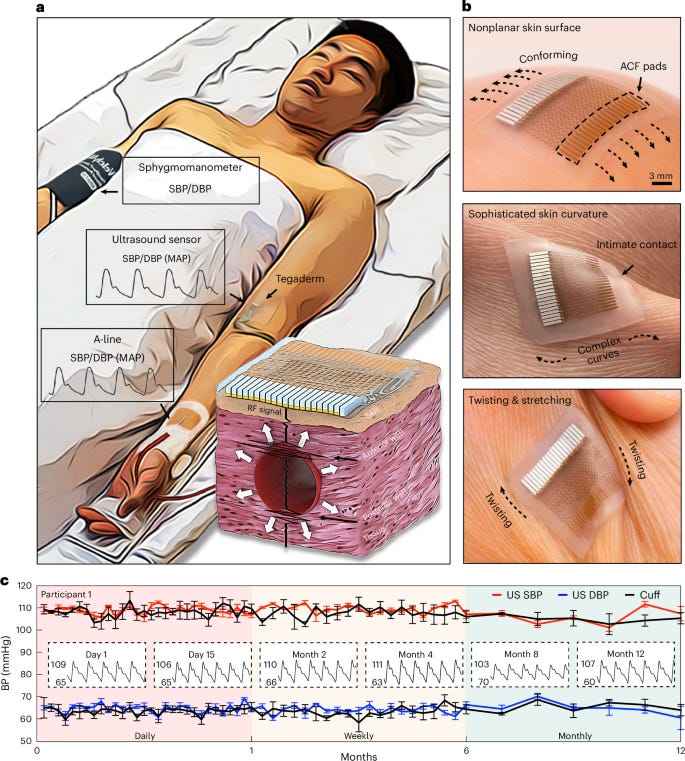
3. Blood Pressure Monitoring. Wearable ultrasound for continuous blood pressure monitoring.
Zhou et al. developed and validated a novel wearable ultrasound sensor for continuous, non-invasive blood pressure monitoring. The device integrates two key technical innovations: a redesigned transducer array optimized for peripheral arteries, and a backing layer that reduces redundant vibrations for more precise arterial wall tracking. The study conducted multiple clinical validations: (1) outpatient testing in 85 participants validated clinical-grade accuracy against standard blood pressure cuffs during postural changes; (2) cardiac catheterization studies in 21 patients demonstrated agreement with arterial line measurements (the clinical gold-standard for continuous BP monitoring); (3) intensive care unit validation in four post-operative patients confirmed measurement stability during 12-hour continuous monitoring; (4) daily activity assessments in seven participants verified measurement consistency during various daily activities; and (5) longitudinal validation in four participants demonstrated sustained calibration accuracy for at least a year. This device addresses key limitations of existing BP monitoring methods by enabling reliable, long-term measurement without the constraints of cuff-based or invasive approaches.
Read Paper | Nature Biomedical Engineering
4. AI Workload Impact. Would the use of AI in radiology practice increase radiologist burnout?
Liu et al. conducted a comprehensive cross-sectional study of 6,726 radiologists across China and found an association between AI use and increased burnout rates among radiologists. The study evaluated burnout across three dimensions: emotional exhaustion, depersonalization, and personal accomplishment. Results showed that radiologists who regularly used AI tools have significantly higher rates of burnout (40.9%) compared to non-AI users (38.6%), exhibiting a significant dose-response association. AI use was significantly associated with emotional exhaustion, and the effect was amplified among radiologists with high workloads or lower AI acceptance. The study challenges previous assumptions about AI's role in reducing radiologist workload, suggesting that current AI implementation may actually contribute to increased stress through elevated consultation volumes and heightened postprocessing demands. For radiologists, the inherently isolated and sedentary nature of their work contributes to higher burnout rates compared with other specialties. AI may further exacerbate these challenges by diminishing opportunities for peer collaboration and patient interaction, while fears of job displacement and uncertainties surrounding AI use heighten stress.
Read Paper | JAMA Network Open
-- Emma Chen, Pranav Rajpurkar & Eric Topol