We are moving our newsletter to Substack for a better experience!
In Week #243 of the Doctor Penguin newsletter, the following papers caught our attention:
1. 3D Biomedical Imaging. The scarcity of labeled 3D biomedical imaging data poses a significant challenge for developing deep learning models. Can we utilize the relatively abundant annotated 2D data to enhance the performance of models designed for 3D volumetric analysis?
Avram et al. developed SLIViT, a deep learning model for analyzing 3D medical imaging data across multiple modalities, including OCT, ultrasound, MRI, and CT. SLIViT leverages knowledge from abundantly annotated 2D medical images to perform effectively on 3D imaging tasks, even with limited 3D training data. The model processes input by vertically concatenating frames of 3D volumes into an 'elongated image,' which is then fed into a ConvNeXt-based feature-map extractor pre-trained on both natural and biomedical 2D labeled images. The entire model, comprising the feature extractor and integrator, is fine-tuned end-to-end for specific 3D imaging tasks. This cross-dimensional transfer learning approach enables SLIViT to generalize across various 3D imaging modalities and medical domains. The study evaluated SLIViT in eight different learning tasks, including classification and regression, using six datasets involving four volumetric imaging modalities. Results showed that SLIViT consistently outperformed existing state-of-the-art models and achieved accuracy comparable to clinical specialists in detecting disease biomarkers, while operating at significantly higher speeds.
Read paper | Nature Biomedical Engineering
2. Biological Aging Marker. A non-invasive approach to measure biological age.
Nusinovici et al. developed RetiPhenoAge, a convolutional neural network-based biological aging marker that predicts a person's biological age from retinal photographs. Unlike most biological aging marker studies that use chronological age as the ground truth, RetiPhenoAge is trained to predict PhenoAge, a blood biomarker of biological age. The model was trained on retinal photographs and blood samples from over 34,000 UK Biobank participants and validated in independent cohorts from Singapore and the USA. RetiPhenoAge demonstrated strong associations with increased risks of all-cause mortality, cardiovascular disease mortality, cancer mortality, and cardiovascular disease events, even after adjusting for confounders. It outperformed other aging biomarkers such as hand grip strength, telomere length, and physical activity. The study identified BMI, smoking status, and diabetes as the most significant contributors to RetiPhenoAge variations. Additionally, RetiPhenoAge showed associations with two genetic loci and several blood glycolysis-related metabolites, including citrate and lactate.
Read Paper | The Lancet Healthy Longevity
3. ECG. Cardiac hypertrophy and dilation, major causes of heart failure, are mainly diagnosed by echocardiography after symptoms appear. Could AI enhance detection of these conditions using only electrocardiogram (ECG) data, which is routinely used in physical examinations?
Zhu et al. developed ECG-Cor-Net, a multi-label deep learning model for early diagnosis of cardiac hypertrophy and dilation using ECGs as a single data source. The model was trained on a large dataset of 90,895 12-lead ECGs from 74,562 patients, with echocardiography serving as the gold standard. ECG-Cor-Net processes both 10-second ECG time series data and 2D waveform images of one heartbeat segment. The 2D images provide a higher-dimensional representation, capturing waveform variations, widths, slopes, and hidden spatial contextual information, while the 1D time series are more sensitive to frequency and interval changes. Results show that ECG-Cor-Net outperforms experienced physicians in detecting left and right ventricular hypertrophy/dilation and atrial enlargement, increasing average sensitivity from 0.270 (physicians) to 0.586, indicating that more than half of the cases could have been diagnosed during routine monitoring. The study also identified four representative ECG leads (I, aVR, V1, and V5) and demonstrated that a simplified model using only these leads performed comparably to the 12-lead model.
Read Paper | NEJM AI
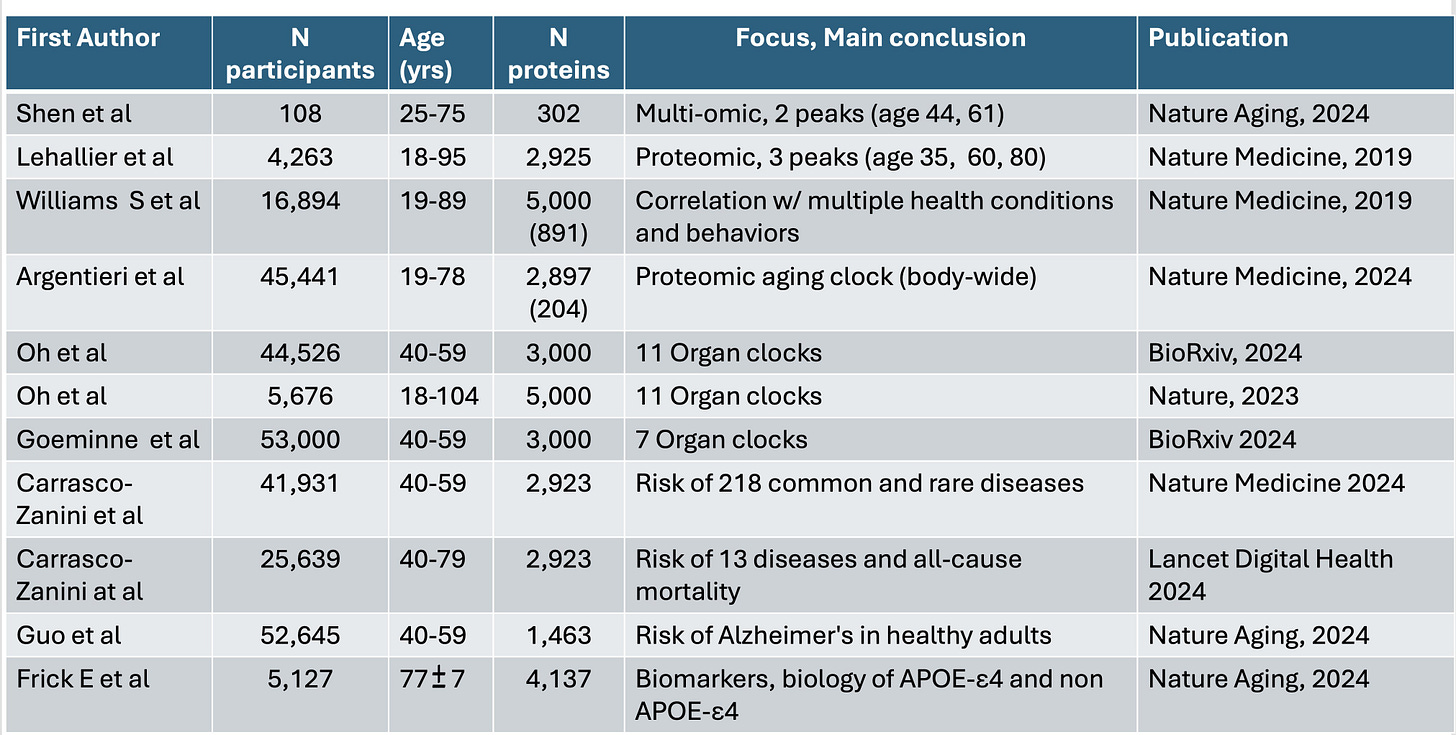
4. Proteomics. Polygenic risk scores, while valuable for assessing disease risk, provide an incomplete picture of human health, as machine learning analysis of proteins and other factors beyond common DNA variants is revolutionizing our understanding of disease foundations.
In this perspective, Topol examines how recent breakthroughs in high-throughput proteomics have transformed our understanding of human health, aging, and disease risk. By analyzing thousands of proteins at both organ-specific and body-wide levels, researchers have developed "aging clocks" and predictive models for multiple diseases and mortality. These proteomic analyses, particularly when integrated with other data types and processed through machine learning algorithms, offer a more dynamic and comprehensive view of human health than genomics alone. While the current cost of comprehensive proteomic analysis remains high—ranging from $500 to $1000 per individual—the development of targeted protein panels for specific risk assessments may significantly reduce expenses, paving the way for broader clinical application.
Read Paper | Science
-- Emma Chen, Pranav Rajpurkar & Eric Topol