We are moving our newsletter to Substack for a better experience!
In Week #242 of the Doctor Penguin newsletter, the following papers caught our attention:
1. Drug Repurposing. Current predictive models for drug-repurposing focus narrowly on diseases with existing drugs. However, this approach overlooks the long tail of diseases with few or no therapies. Therefore, models with zero-shot drug-repurposing prediction ability may be more clinically useful by potentially identifying treatments for understudied diseases.
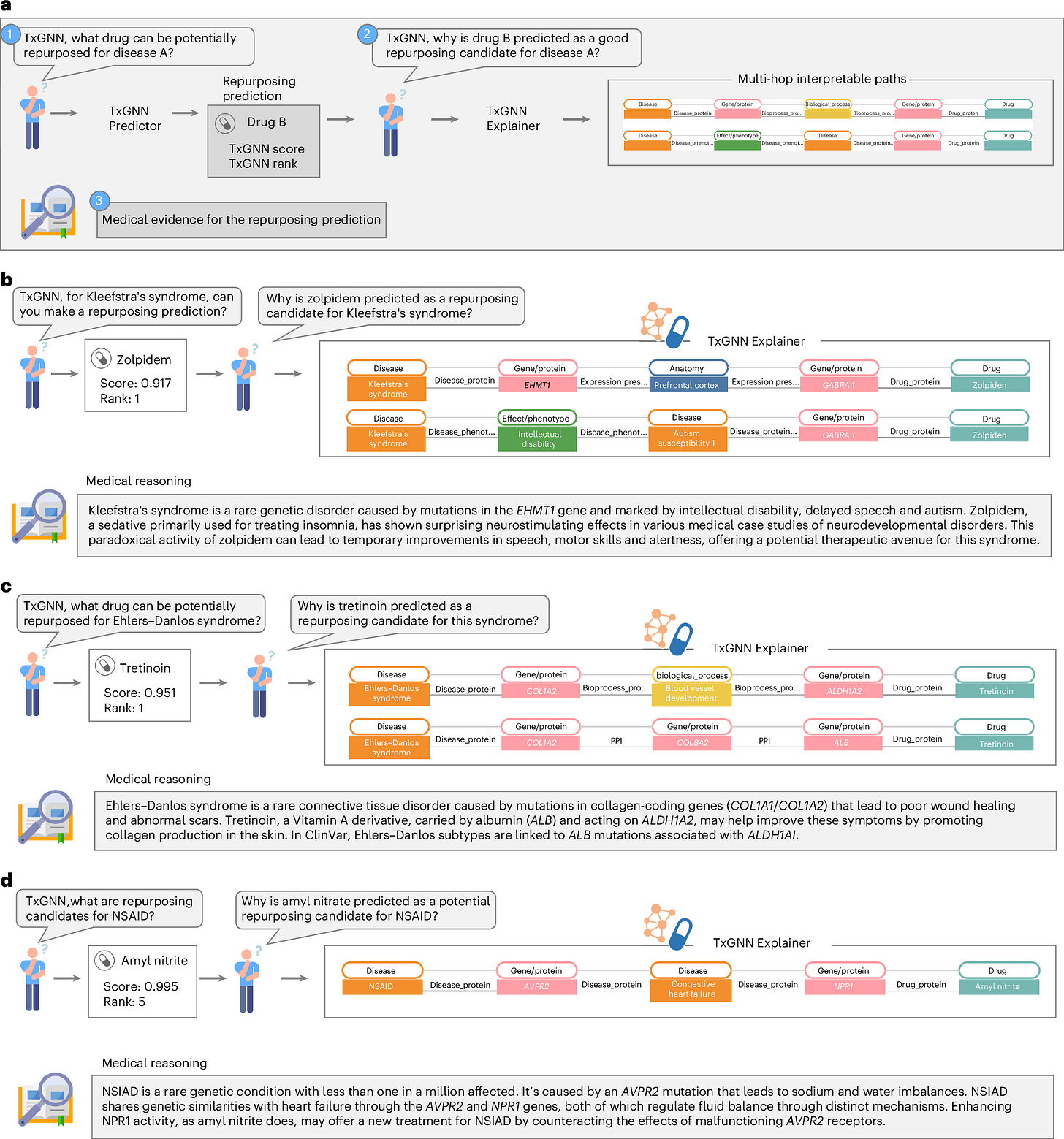
Huang et al. introduce TxGNN, a graph foundation model for zero-shot drug repurposing that can identify therapeutic candidates for diseases with limited or no existing treatments. TxGNN leverages a comprehensive heterogeneous biological knowledge graph comprising 10 types of nodes (e.g., diseases, drugs, genes/proteins) and 29 types of undirected edges (e.g., drug-disease relationships, drug-protein interactions). TxGNN predicts both indications and contraindications for disease-drug pairs, while also generating interpretable explanations through multi-hop paths. For instance, when suggesting amyl nitrite for the rare condition NSIAD, TxGNN points out the connection between NSIAD and amyl nitrite through congestive heart failure, by exploring gene interactions (AVPR2 and NPR1) that regulate electrolyte balance (i.e., NSIAD → AVPR2 gene → Congestive heart failure → NPR1 gene → Amyl nitrite). Trained on 17,080 diseases and 7,957 drugs, TxGNN's predictions have demonstrated alignment with off-label prescriptions in a large healthcare system. Moreover, a pilot human evaluation revealed that medical experts could more effectively assess predicted drugs using the multi-hop explanations compared to alternative visualization methods, underscoring the model's potential to enhance drug repurposing efforts.
Read paper | Nature Medicine
2. ECG. Myocardial blood flow (MBF) and myocardial flow reserve (MFR) are independent parameters for risk stratification in patients with suspected coronary artery disease. Positron emission tomography (PET) can accurately measure MBF and MFR, but is expensive and less accessible. Predicting MFR when PET myocardial perfusion imaging is unavailable would offer valuable information for preventative and proactive medical care.
Alahdab et al. developed a machine learning model to predict MBF and MFR from patients' ECGs. The model combines ECG data from standard 12-lead ECGs with clinical features such as age and gender. It employs a Binary Gray Wolf Optimizer (BGWO), a swarm intelligence algorithm that simulates gray wolf hunting to iteratively select important ECG features based on the current best three subsets of features, gradually converging on the optimal feature selection that minimizes both the number of features and prediction error. The selected ECG features are then fused with clinical data using Discriminant Correlation Analysis. Trained on 3,600+ paired ECG and PET myocardial perfusion imaging scans, the model predicted impaired MFR with a sensitivity of 73.3% and a specificity of 76.7%. The predicted MFR correlated well with the "ground truth" PET MPI-derived MFR. The model also demonstrated the ability to predict patients' coronary flow and, more importantly, future adverse cardiac events. This approach could identify high-risk patients using widely available 12-lead ECGs, potentially reducing reliance on costly, less accessible PET scans.
Read Paper | Cell Reports Medicine
3. Insurance Reimbursement. Only 37% of U.S. adults can cover an unexpected $400 expense without borrowing or selling assets. When AI tools recommend tests not yet covered by insurance, patients may face new financial burdens if their clinicians follow these recommendations.
In this perspective, Jain et al. provide a brief overview of the process for obtaining insurance coverage for tests or procedures and discuss the issue of insurance coverage for AI-recommended diagnostic tests. While identifying at-risk patients missed by traditional assessments is a key benefit of AI, those lacking traditional markers may not receive insurance coverage for subsequent testing, monitoring, or treatment. Consequently, even when AI tools perform equally well across patient subgroups, the benefits may not be equitably distributed. To address this, the authors advise healthcare organizations to evaluate AI tools for financial and equity implications before deployment, understand insurers' coverage rules, and consider offering low-cost or free initial confirmatory testing since follow-up care may be covered by insurance once the diagnosis is confirmed. For insurers, they recommend developing new standards for coverage decisions based on AI tool recommendations and creating processes to rapidly re-evaluate these decisions as AI evolves.
Read Paper | The New England Journal of Medicine
-- Emma Chen, Pranav Rajpurkar & Eric Topol